Chapter 2.0: Biological Models of Substance Misuse, Pharmacokinetics, and Psychopharmacology Principles
Ch. 2.2: Neurobiology and Substance Use
The biological realm of substance use, substance misuse, substance use disorder includes neuroanatomy, neurophysiology, and neurochemistry. Neurobiology investigators are developing increasingly complex, detailed, functional maps of the various regions of the brain involved in substance use, misuse, and SUD. These maps show how the brain’s powerful pain, pleasure, reward, and memory systems interact in the process of substance use becoming a substance use disorder—and how psychological learning principles operate at a neurobiological level. This knowledge also helps us understand how difficult it can be to recover from SUD/addiction and why the age/stage of development when substance use is initiated matters in the outcomes.
Learning about the neurochemistry actions of specific substances in neurophysiology also helps us understand the actions of different substances on the brain-behavior link. Here we will look at neurotransmitters and their role in the experience of substance use/misuse. This knowledge helps investigators develop intervention strategies for treatment, relapse prevention, and even preventing the development of substance use disorders. These biologically based strategies include medications and the use of mindfulness meditation and neurofeedback approaches.
Neuroanatomy and Function
The structure and organization of the central nervous system (CNS) has been studied for a very long time. Current technologies such as functional magnetic resonance imaging (fMRI) help develop our understanding of how different areas of the brain are involved in specific experiences or behaviors, and how exposure to different events or substances might affect specific brain areas and functions. There are certain brain regions identified as having a significant role in the development of SUD. In addition, the brain-behavior link is influenced by and influences the autonomic nervous system (ANS) which controls many bodily functions outside of conscious thought (e.g., heart and breathing rate, blood pressure, and others). Many psychoactive substances not only affect the “mind,” they also affect other organs and systems, including the ANS. When we examine different types of substances, you will see how the health and functioning in other systems is also affected by psychoactive substances.
Limbic system. The limbic system helps regulate basic drives, emotions, arousal and attentiveness (Begun & Brown, 2014). As such, it helps coordinate the neurobiological experience of stress and the reward system triggered by exposure to drugs. The amygdala and nucleus accumbens are two important components of the limbic system with regard to substance misuse (Logrip, Zorilla, & Koob, 2012), along with the hippocampus.
Amygdala. The amygdala plays a central role in emotional responses to internal and external stimuli—pleasure, fear, anxiety, and anger included. It is central to survival as it manages the “fight or flight” response to perceived threats in the environment, which in turn, is related to the experience of stress. The amygdala is also responsible for the emotional content of our memories—determining not only which experiences related to pain and pleasure become encoded into memory, but also the emotional values attached to the formation of new memories. This area is one target of anti-anxiety medications but is also influenced by the actions of various substances that might be misused.
Hippocampus. The hippocampus is involved in memory, as well, particularly memories related to traumatic events and learned responses to environmental cues. This becomes an important factor in the experience of cravings triggered by environmental cues, as well as the relationship between trauma and substance misuse/SUD.
Nucleus accumbens. The nucleus accumbens is part of what is called the mesolimbic dopamine system—it is highly involved in positive reinforcement, leading to a person anticipating reward with repetition of the previously positively reinforced behavior. Thus, if a substance increases the release of dopamine in this area, the person comes to anticipate positive reinforcement again with future use. The amount of dopamine increase can far exceed what natural behaviors trigger (eating or sex, for example) and the amount of dopamine directly relates to the degree of pleasure experienced (Volkow et al., 2010). Thus, a person may come to preferentially engage in substance use over naturally rewarding behaviors (like eating or sex).
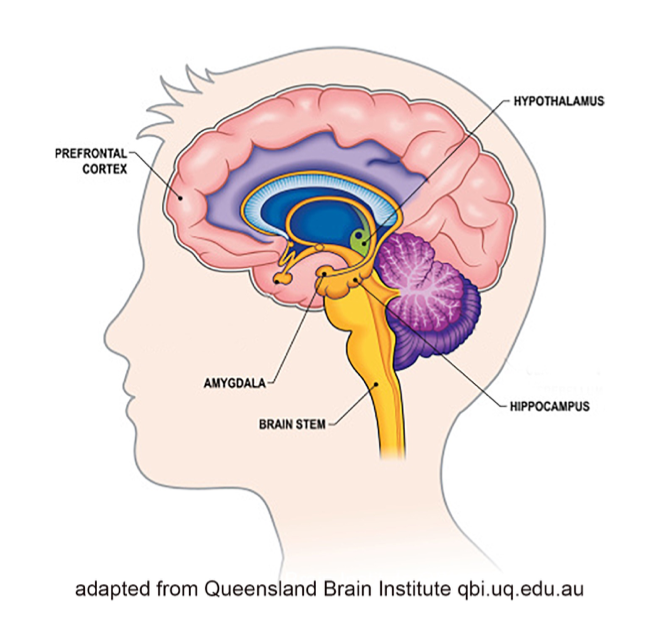
Prefrontal cortex. The prefrontal cortex is linked to the amygdala—they communicate directly. This is a “thinking” part of the brain where functions like cognition, comprehension, concentration, reasoning, planning, and initiating goal-directed behavior takes place (Giancola & Tarter, 1999). The area is responsible for a person’s intentional responses to the experiences the amygdala sends forward. For example, the conscious decision to initially engage in substance use. This part of the brain is also highly susceptible to alteration, even damage, from exposure to many substances, reducing its capacity to mediate responses triggered by the amygdala (Begun & Brown, 2014). As a result, a person might be less able to dampen the amygdala’s push to action, acting more impulsively than thoughtfully/intentionally, especially in terms of relapse responses. The paradox is that the very area responsible for helping someone control substance misuse is an area impaired by substance misuse (Azmitia, 2001).
Changes in Brain Function
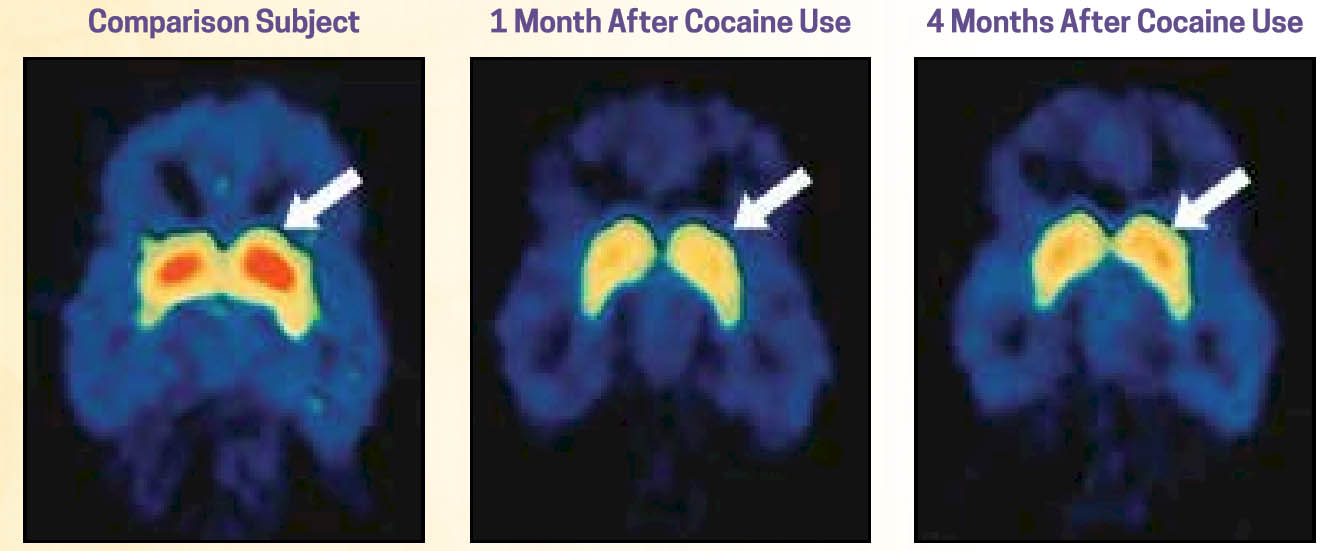
Changes in how these areas of the brain function following exposure to certain substances, particularly heavy, repeated (chronic) substance misuse, are evident in fMRI (functional magnetic resonance imaging) scans. Additionally, changes remain evident well after the substance use ceases—although the brain does begin to recover and return to more normal appearing functioning. In the following sequence of brain scans, the image on the left is of a person who has not engaged in cocaine use (the “normal” control brain). The other two scans represent a person who has a history of cocaine use disorder 1 month and 4 months after use has ceased. The areas in red represent the density of dopamine receptors in an area of the brain (striatum) responsible for various cognitive functions, including a role in planfulness and self-control—low dopamine receptor density in this region was associated with loss of control. As you can see in these images, there is some improvement at 4 months post-use, but function has not returned to normal (images from NIDA, 2018).
Developmental Impact
A great deal of attention to the developmental effects of exposure to alcohol and other drugs has been directed to two life periods: prenatal exposure and substance use during adolescence/emerging adulthood. These two developmental periods have an important commonality: these are periods when the brain is naturally undergoing rapid developmental growth or change. Thus, introducing substances that affect the brain can have more pronounced, amplified, and pervasive long-term effects.
Prenatal exposure. That alcohol exposure during fetal development can cause permanent damage to the brain and other organs has long been recognized, and fetal alcohol syndrome (FAS) was clearly identified as a possible outcome during the 1970s (Jones & Smith, 1973). Subsequent work has led to expansion of the definition and diagnosis of possible prenatal alcohol exposure (PAE) outcomes to reflect a spectrum referred to as fetal alcohol spectrum disorders (FASD) (Streissguth et al., 2000). FASD includes the syndrome (FAS), as well as alcohol-related neurodevelopmental disorder (ARND) and alcohol-related birth defects (ARBD). [Note that ARBD is also used to describe alcohol-related brain damage or ARBI for alcohol-related brain injury experienced by individuals later in life whose drinking patterns leads to brain injury, or ARBI for alcohol-related brain injury.] FASD is perhaps best understood as a “whole-body” diagnosis, as individuals with FASD experience a wide range of health and mental health conditions throughout life (Himmelreich, Lutke, & Hargrove, in press).
The effects of prenatal exposure to other substances is less well understood. Neonatal abstinence syndrome (NAS) is a known consequence experienced by many, but not all, infants prenatally exposed to opiates/opioids (Reber, Schlegel, Braswell, & Shepherd, in press). NAS concerns the infant’s experience of withdrawal from the substances previously circulating from the mother through the placenta and abruptly stopped with birth. The long-term complications of NAS may, but do not necessarily, include neurocognitive and behavioral effects (Reber et al., in press). We will learn more about the known and possible effects of prenatal exposure to different types of substances as we learn about each in Part 2 of our course. It is important to know that many effects of prenatal exposure to alcohol or other substances do not appear right away at birth; some do not appear until children enter school or face increasingly demanding social and cognitive challenges which their brains are ill-equipped to handle. To minimize the negative developmental impacts of prenatal exposure and maximize developmental potential, early diagnosis and intervention is optimal (Loock, Elliott, & Cox, in press)—ideally, involving integrated teams of social work, medicine, nursing, physical therapy, occupational therapy, nutrition, and early education professionals.
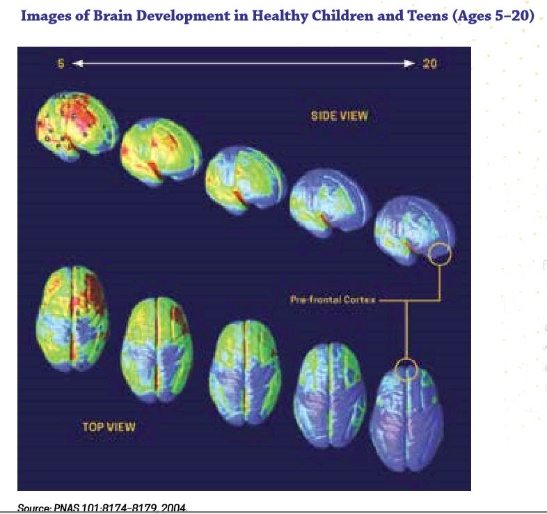
Adolescent/emerging adulthood exposure. Shortly before and during puberty the human brain begins to undergo dramatic remodeling changes. The physical changes, to a large extent, involve reorganization of the connections between neurons and communication pathways between brain regions, particularly in the prefrontal cortex. On one hand, a great deal of neuron “pruning” takes place, trimming out a great many underused or unused connections between neurons. On the other hand, myelination of existing neurons enhances connections between neurons that remain linked (Siegel, 2014). These two processes make the brain more efficient, better integrated, and capable of higher order functioning, but do not happen evenly and at the same time in all brain regions. The result is emotional functioning similar to that of adults but cognitive functioning that is as yet under-developed in terms of decision making, inhibitory control, planning, and working memory (Meredith & Squeglia, in press). Additionally, the adolescent brain is characterized by “heightened reward sensitivity and underdeveloped cognitive control that contribute to risky behaviors, including escalating substance use” (Meredith & Squeglia, in press). Heightened reward sensitivity suggests that the positive reinforcement experienced with substance use is experienced as more intensely positive (stronger reinforcement) than what is experienced by individuals later in life. The brain revision process normally tapers off from about ages 20 to 25. This image (from NIDA, 2018) shows how the concentration of grey matter shifts from age 5 to 20—the shift from yellow to blue in these images.
Thus, the brain is quite sensitive to developmental consequences of exposure to psychoactive/psychotropic substances up until age 25. The use of alcohol or other substances during these years can have profound, lasting effects on the still-developing brain; effects which have significant implications for how people think, behave, and feel, as well as for susceptibility to developing substance use disorders later in life. “In studies of drug use, an earlier age at which drug use was initiated is consistently related to a greater level of later drug-related problems,” (Hawkins et al., 1997, p. 281), making a delay in age of substance use initiation an important prevention strategy. Chances of developing severe substance use disorders is higher among individuals whose substance use began before age 15 years; “the biggest reduction in risk with deferred age of onset occurs when first use is postponed beyond age 15” (Robins & Przybeck, 1975, p. 184). Alcohol dependence was found to be four times more likely and alcohol abuse twice as likely among individuals whose age of drinking onset was before age 15 compared to individuals whose onset was delayed to age 21: “Overall, the risk for alcohol dependence decreased by 14 percent with each increasing year of age of drinking onset” (NIAAA, 1998). Deficits in adolescent brain functions and cognitive performance were observed with as little as 20 drinks per month, particularly if binge drinking was involved (Squeglia, Jacobus, & Tapert, 2009); some but not as great a level of divergence from their peers was detected with marijuana use. Finally, consider that a person’s overall health and development may be affected by poor nutrition, physical trauma or injury, or exposure to diseases that often accompany substance misuse.
Neurochemistry/Neurophysiology and Function
Previously we explored what was happening at the level of brain regions. Now we turn attention to what is happening at a more microscopic level—neurons. As you may know from your previous education, the central nervous system (CNS) is comprised of about 86 billion nerve cells, called neurons, and about an equal number of glial cells that provide the energy neurons need to function (BrainFacts/SfN, https://www.brainfacts.org/in-the-lab/meet-the-researcher/2018/how-many-neurons-are-in-the-brain-120418). It makes sense to consider neurons and glial cells at this microscopic level because they are the building blocks of the brain regions previously discussed as playing key roles in substance use, substance misuse, and substance use disorder.
Neuron activity. The neurochemistry of substance use operates largely at two points. The first concerns the glial cells and how much energy they can provide to neurons—the loss of glial cells or impeding their ability to provide energy has a negative impact on neuronal activity. The second concerns the ways that neurons communicate. Neurons physically pass neurotransmitters (molecules of naturally occurring brain chemicals) between each other as their mechanism for communication. Whether one neuron activates the next one depends on whether neurotransmitters are sent, whether those neurotransmitters are received by the next neuron, the amount of neurotransmitter sent and received, and the rate at which the neurotransmitters are reabsorbed after a “message” has been sent.
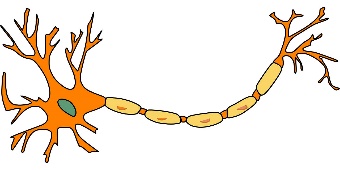
Neurotransmitters. A neuron’s neurotransmitter molecules are contained in packets called vesicles, located in the terminal area of a neuron’s axon—the area that comes into close contact with the neighboring neurons (see Figure 3-2). The space between the neurons is the synapse/synaptic cleft. This space between neurons is where neurotransmitters are released to work their changes. The “sending” neuron is the presynaptic neuron, while the receiving neuron is the postsynaptic neuron.
Figure 3-2. Neurons and how they communicate
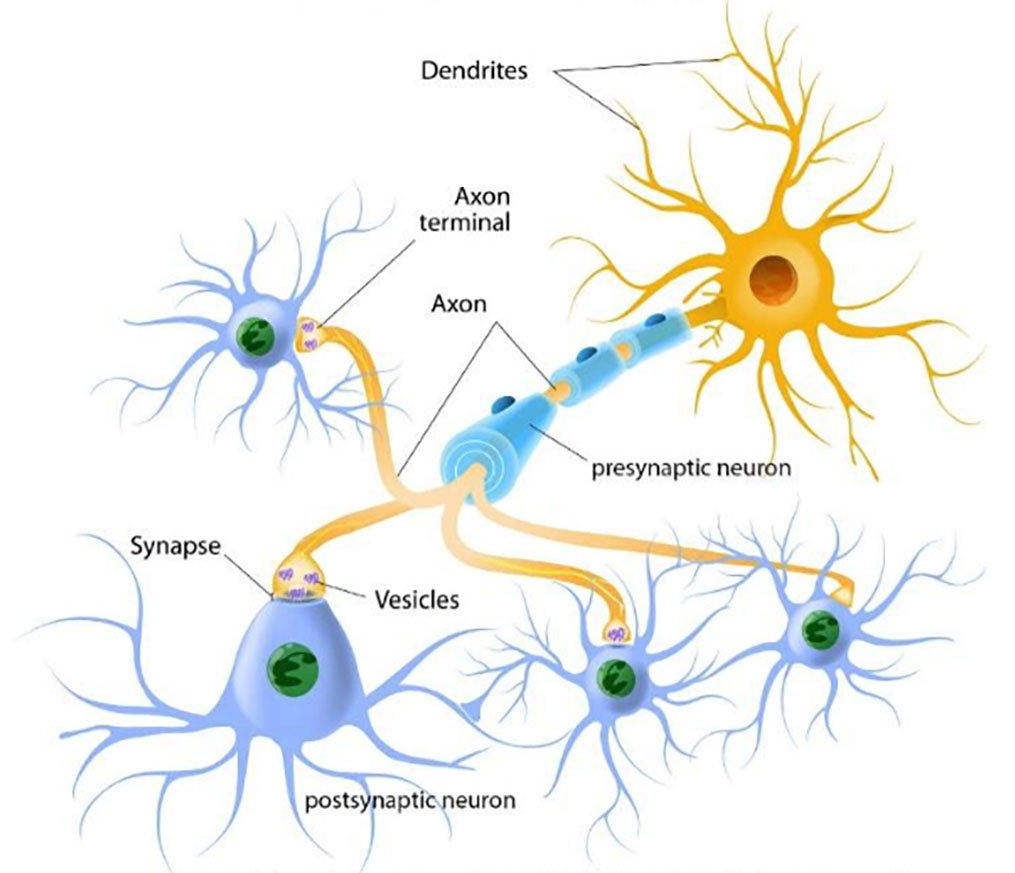
The presynaptic (first) neuron releases neurotransmitter molecules (stored in the vesicles) into the synapse between it and the postsynaptic (next) neuron. The postsynaptic neuron “receives” the neurotransmitter chemical if it has the right neurotransmitter receptors—kind of like a lock and key system. Neurotransmitters need the right receptors in order to “dock” and influence the postsynaptic neuron: if the right receptors are available, the neurotransmitter delivers the message but if the right receptors are not available, the neurotransmitter has no effect and just sits in the synapse. If the message is received by the postsynaptic neuron, it can now pass the message along to the next neurons in line. In the meantime, transporters retrieve and return the “used” neurotransmitter molecules back into the presynaptic neuron’s vesicles in preparation for sending a future message (see Figure 3-3). If a neuron has released its neurotransmitter molecules, it cannot send new messages until the supply has been restocked.
Figure 3-3. Diagram of neurotransmission at the synapse (from science.education.nih.gov/supplements/webversions/BrainAddiction/other/)
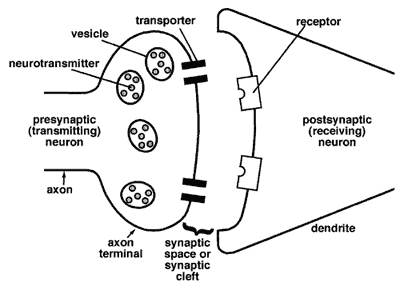
If the postsynaptic neuron’s receptors are already filled, then the sent message will not be received—the neurotransmitters are blocked. This is how some drugs work—they occupy the receptor sites, thereby blocking messages between neurons. Other drugs work to reduce or increase receptor site sensitivity to the neurotransmitters. Still others work to influence the amount of neurotransmitter released into the synapse or affect the transporters’ work in returning the neurotransmitter molecules to the vesicles.
Types of neurotransmitters. Different types of neurotransmitters have different impacts. For example, some play a more excitatory role, while others play a more inhibitory role. Excitatory neurotransmitters increase the likelihood that the receiving (postsynaptic) neuron will be triggered into activity; inhibitory neurotransmitters suppress this kind of activity. Most types of neurotransmitter are either excitatory or inhibitory; a few can be either (e.g., dopamine). Different types of neurotransmitters are more concentrated in specific brain regions—while they may be distributed throughout the brain, they are not evenly distributed. This is why different substances “trigger” certain brain regions more than others—their effects are produced through their influence on the neurotransmitter communication processes and those neurotransmitters are more concentrated in certain regions.
Several types of neurotransmitter are known to play a role in the development, maintenance, and recovery from alcohol or other substance use disorders. Presented alphabetically, these neurotransmitters (and closely related neuropeptides) include:
- dopamine has both excitatory and inhibitory effects, depending on the nature of the receptor sites involved, is associated with the brain’s reward systems, and is increased to abnormal levels by substances such as alcohol, cocaine, and heroin (influencing their addictive potential);
- endorphins & enkaphlins are two neuropeptides (rather than neurotransmitters) that play a role in producing some of the rewarding effects experienced with the use of alcohol and some other substances—endorphins relate to opiate receptors causing an analgesic (pain control) effect and enkephalins are similar to endorphins;
- epinephrine is an excitatory neurotransmitter (also called adrenaline) involved in the “fight or flight” response;
- GABA (gamma-aminobutyric acid) is an inhibitory neurotransmitter widely distributed throughout the brain and plays a critical role in alcohol misuse and alcohol use disorder (and possibly other substances) because alcohol increases the effect of GABA contributing to feeling more calm, relaxed, and even sleepy;
- glutamate is the most common neurotransmitter found in the human CNS, is excitatory, plays a key role in regulating attention and arousal, and typically acts in opposition to GABA;
- norepinephrine acts in opposition to epinephrine, as an inhibitory agent, to control “fight or flight” functions stimulated by epinephrine (also called noradrenaline);
- serotonin is an inhibitory neurotransmitter that helps regulate many functions (sleep, cravings, and pain control, among others) and emotional states, off-setting the effects of excitatory neurotransmitters.
Several things are very important to understand about neurotransmitters and the system of communication in which they are involved:
- We used to believe that each neuron could only release one type of neurotransmitter. More recent research indicates that in many cases the same neuron can release two and possibly more types depending on the frequency of the stimulation it receives—at one frequency it might release one type of neurotransmitter, at another frequency it might release a different type.
- Most neurotransmitters occur naturally as important chemicals in other parts of the body (including the peripheral nervous system and other organs) where they have other health-related functions, not just in the brain (central nervous system). For example, the human body naturally has opioid and cannabinoid receptors that are meant to respond to naturally occurring (endogenous) chemicals to control pain, reward certain life-supporting behaviors, and influence learning and memory. These receptors are also responsive to introduced chemicals (exogenous) which are often introduced in much higher doses than naturally occur—from using cannabis/marijuana or opioid drugs. Opioid receptors are also involved in responses to alcohol.
- Neurotransmitter release is triggered by many natural behaviors, not just by alcohol and other substances. For example, dopamine release is involved in the natural reward systems associated with food, sex, humor, pair-bonding (mates), listening to music, and video games. The addictive potential of a psychoactive drug increases when the concentration of dopamine released is higher compared to what is released by natural behaviors (Johnson, 2014).
- Fast uptake of a drug, for example getting it to the brain by injection rather than ingesting it orally, produces a stronger “high” and therefore a greater potential for addiction. This is because more dopamine is released at once, so it is more rewarding (Volkow et al., 2010).
Homeostasis
One hallmark of the human brain is its adaptability (neuroplasticity), whereby its various functions adjust to conditions in order to maintain overall balance or homeostasis. This adaptability gives rise to acquired tolerance when a substance (or type of substance) is used repeatedly over time. Homeostasis plays a role in the development of tolerance, as well as the biological basis of the substance withdrawal experience. In addition, the age at which the brain becomes exposed to substances matters.
Acquired tolerance. Acquired tolerance is defined as a person requiring higher doses of a substance (or type of substance) to achieve the same effects or experiencing lesser effects (even withdrawal) when the same dose is used if the substances have been used repeatedly over time. Let’s consider what is happening at a neurochemical level. When a person uses a great deal of alcohol often over time, the brain begins to adapt to the presence of the alcohol and its effect on GABA. In attempting to reacquire a state of homeostasis, the brain boosts its arousal systems (glutamate) to offset the overly inhibitory impact of the extra GABA triggered by the alcohol. This is called upregulation of the glutamate system—additionally activating the system that produces glutamate. In addition, the brain may begin to control the amount of GABA through downregulation of the GABA system—suppressing the system that produces GABA. In other words, two things are going on to offset the effects of chronic alcohol exposure: downregulating GABA and upregulating glutamate. This means that, in order to experience the same effects at the same level, a person needs to take even more alcohol to boost the GABA even more. This internal neurophysiological teeter-totter continues to see-saw over time.
Experience of withdrawal. At this point, you have developed a basic understanding of how neurotransmitters and homeostasis play a role in the development of a substance use disorder. Up until this point, we have been exploring what happens when the brain is exposed to certain substances. Now, let’s look at the other side of the coin: what happens when the brain is no longer exposed to substances to which it has grown accustomed. Remember that the brain has adapted to the chronic presence of the substance (alcohol, in our example) by downregulating GABA and upregulating glutamate systems (see the “Tolerance” section above). Withdrawing the substance (alcohol) means that the GABA and glutamate are going to be out of balance for a while, at least until the GABA begins to upregulate again and the glutamate to downregulate, re-acquiring a state of homeostasis without alcohol being present. The withdrawal of substances can result in the experience of withdrawal symptoms—an experience that may be intense (even potentially deadly) and prolonged. Elsewhere in this text you will learn more about why withdrawal symptoms might make a difference in a maintaining a “quit” attempt or relapsing to using substances again.
We can draw from content presented in articles published by Koob and Simon (2009) and Trevisan et al (1998). They tell us that:
- A decrease in dopamine or serotonin contributes to the experience of dysphoria and anhedonia. Dysphoria is the experience of a profound sense of unease, unhappiness, and general dissatisfaction, often associated with major depression and anxiety. Anhedonia refers to a lessening or inability to experience pleasure. Thus, removing substances that stimulated dopamine or serotonin activity can have these effects.A decrease in GABA contributes to the experience of anxiety, even panic attacks, due to the resulting nervous system hyperactivity. An increase in glutamate contributes to hyperexcitability. Thus, removing substances that affected GABA and/or glutamate activitiy can have these effects.
- An increase in norepinephrine contributes to the experience of stress. Thus, removing substances that affect epinephrine and/or norepinephrine can have this effect.
Why does this matter? These negative emotional and psychological states make it difficult to sustain motivation to avoid using alcohol or other substances and contribute to the pressure a person might feel to relapse into using again. Depending on the nature of the substances involved, withdrawal may lead to decreased dopamine, serotonin, or GABA, as well as increased norepinephrine or glutamate. Knowing about these links between neurotransmitter changes during prolonged withdrawal from using a substance contributed to the development of several medications to help manage these negative experiences and perhaps help a person sustain a “quit” attempt over time (pharmacotherapy). Another reason this matters is that during withdrawal and early recovery from many types of substance use disorders, the risk for suicide is greater than in the general population because of these brain-behavior processes.

